2021/04/30
Successful fabrication of microfluidic devices using 3D hydrogel thin film structures- Achievement opens possibility of creation of artificial cell culture models (organs-on-a-chip) -
- Achievement opens possibility of creation of artificial cell culture models (organs-on-a-chip) -
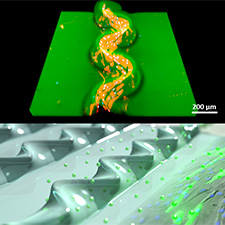
NTT Corporation (NTT) has succeeded in fabricating microfluidic devices that can mimic behaviors in in vivo environments such as shape changes and molecular permeability by deforming highly biocompatible hydrogel thin film*1 to tubes on a rigid substrate and using them as micro flow channels. Producing a cellular culture environment similar to an in vivo environment is critical for artificially reproducing physiological tissues such as organs. NTT has succeeded in developing fundamental technologies that enable cell culture by creating a near in vivo environment on a rigid substrate. This achievement is expected to lead to the creation of culture substrates for cell biology and organs-on-a-chip*2.
NTT researchers discovered that polyacrylamide gel*3, a hydrogel widely used as a biomedical material, can be formed into a 3D structure based on the buckling delamination*4 principle by creating an adhered/non-adhered pattern on a glass substrate. The structure is formed spontaneously due to the swelling force when the hydrogel absorbs water. It is also possible to reverse the structure by reducing the water content in the hydrogel through drying. Because swellability is a general property of hydrogels, the structuring method developed here can be universally applied to a variety of hydrogels.
This technique can create structures with a variety of 3D shapes based on the hydrogel's hardness and thickness and the delamination pattern. The structures can be fabricated over a large surface area with excellent reproducibility. NTT researchers demonstrated the creation of a flow channel structure lifted from hydrogel thin film based on a linear delamination pattern. The structure can function as a microfluidic device into which air or fluid is injected. By adjusting the external application of pressure to the hydrogel flow channel, large deformation of the flow channel and periodic pulsating deformation, characteristics similar to in vivo deformation behavior, were achieved. In addition, NTT researchers succeeded in long-term culture of myoblasts*5 within and outside the wall of the flow channel. They also succeed in creating 3D physiology-mimicking structures along flow channels and achieving localized drug stimulation of cells cultured on the outer surface of a flow channel. The knowledge acquired from these achievements promises to lead to the development of fundamental technologies for stem cell-based regenerative medicine, organs-on-a-chip for drug screening, and models of artificial organs necessary for the design of NTT's Bio Digital Twin*6 technology.
These results were published in the June 15, 2019, issue of the American journal ACS Applied Materials & Interfaces and the April 6, 2021, issue of the British journal Lab on a Chip.
=> Molecular and Bio Science Research Group
Background of research
There is growing demand for technologies for artificially reproducing advanced physiological functions such as organ functions through 3D cell assembly in fields such as cellular biology, regenerative medicine, and drug discovery. To achieve the expression of cells' natural functions experimentally, it is critical to achieve in vitro environment similar to in vivo cellular environment. In particular, scaffold materials for cells must meet the following requirements: (1) the ability to control its shape in 3D, (2) the ability to reproduce stimuli such as sliding and stretching similar to in vivo conditions, and (3) permeability of molecules such as growth factors*7 and cellular waste products. A group of materials satisfying these requires is hydrogels, which are highly biocompatible. Hydrogels are soft materials in which a large amount of water is retained in crosslinked polymer chains. Because they exhibit permeability characteristics similar to those of biomaterials in our own bodies such as organs and cartilage, they are being widely researched as scaffolds for cell culture. However, because in general hydrogels are fragile and rupture easily, fabricating complex 3D structures or freestanding hollow structures on the micrometer scale using hydrogel alone and applying mechanical stimuli has been considered difficult. In addition, there is the problem of supporting large volume changes on a solid substrate because hydrogels absorb water and swell.
Advances in fabrication technologies in recent years have led to techniques for forming 3D hydrogel structures. However, fabrication of thin film tubes that deform significantly, as blood vessels and the intestinal tract do, has been technically difficult. The materials used also had limitations. If a method that allows use of a wide range of hydrogel thin films with various functions to form versatile 3D structures can be established, it will be possible to fabricate structures similar to those found in physiological organs with properties of high stretchability, permeability, and biocompatibility. A cell culture substrate formed by this method, mimicking an in vivo environment, is expected to lead to applications such as organ-on-a-chip, regenerative medicine, and drug screening.
Research results
In this research, we focused on the swelling of hydrogel, which had been difficult to control, and solved the problems described above by investigating structuring methods that exploit swelling. We fabricated a 3D structure based on buckling delamination by attaching a thin film of polyacrylamide gel, which is stretchable, permeable, and biocompatible, in a pattern on a glass substrate and inducing swelling in water (Fig. 1, Video 1). The obtained structure can be reversibly changed according to the degree of swelling. Also, the pattern of adhesion between the hydrogel and glass substrate can be controlled easily over a large surface area by using general-purpose lithography techniques. As an example of this technique, we successfully fabricated a flow channel structure from hydrogel thin film. The linear pattern of this structure floats from its linear delamination pattern. We demonstrated that this flow channel structure can be connected to a pump via a feed tube and used as a microfluidic device capable of injecting air or liquid. It is possible to control the deformation behavior of the hydrogel flow channel of this device by adjusting the pressure of the pump. Furthermore, we have also succeeded in long-term culture of cells inside and outside flow channels. Successful cell cultivation on the inner wall of the channel is expected to lead to the development of template technologies for the formation of artificial organs. Meanwhile, taking advantage of the hydrogel's permeability, cell culture on the outer surface of flow channels allows localized chemical stimulation of externally cultured cells through injection of drugs into flow channels.
Key points of technology: Structuring of hydrogel thin film using buckling delamination and application to fluidic devices.
- Technological point (1)
Buckling delamination-based 3D structuring and control of hydrogel thin film
The position and detailed shape of the 3D structure formed by buckling delamination can be controlled by the delamination pattern and the hardness and thickness of the hydrogel thin film. For example, by setting the delamination pattern as a stripe pattern or honeycomb pattern, linear or complex merged flow channels can be fabricated (Fig. 2(a)). Also, by changing the width of stripes in the delamination pattern and the hardness of the hydrogel, the structure can be controlled to become various 3D shapes such as linear, snake, and double-period snake shapes (Fig. 2(b)). This method can be applied to a variety of hydrogels. We have verified that by using stimuli-responsive gels, whose degree of swelling changes in response to stimuli, we can dynamically and reversibly control 3D structures with external stimuli. For example, we succeeded in achieving fine control of a hydrogel structure by using an electrolyte-responsive gel and switching of hydrogel structure by using a thermo-responsive gel (Fig. 3, Video 2). - Technological point (2)
Application to microfluidic devices
A flow channel structure covering the hydrogel thin film and glass substrate can be fabricated by designing a linear delamination pattern. By connecting a feeding tube to this flow channel structure using water-resistant putty and an affixing jig, the structure can be used as a microfluidic device capable of pumping liquid under pressure (Fig. 4(a)). We fabricated two types of devices: a thin film device that deforms significantly under pressure, and a stacked device for which deformation is adjusted by coating the outer surface of the structure with gel (Fig. 4(b)). By changing the pressure applied from the outside, we successfully reproduced repeated behaviors of significant deformation and periodic pulsating deformation. We thus demonstrated functions that are capable of mimicking in vivo deformation by using microfluidic devices (Video 3). - Technological point (3)
Long-term cultivation of cells within and outside of flow channels and localized stimulation due to drug permeability
Because hydrogels have high biocompatibility, it is possible to use hydrogel flow channels as scaffolds and carry out long-term cell culture. When myoblasts were cultured within a flow channel, the spread of cells along the inner wall of the flow channel was observed (Fig. 5(a), Video 4). Also, when myoblasts were cultured on the aforementioned stacked device and acetylcholine*8 was injected into the flow channel, it was observed that the drug reached outside the flow channel due the hydrogel's permeability. We thus verified the possibility of localized chemical stimulation (Fig. 5(b)). Fluorescent visualization of the drug-stimulated cells indicates that they were stimulated in a wave pattern directly on top of the flow channel (Video 5).
Future developments
In this research, we developed a technique to freely form 3D structures based on buckling delamination by inducing swelling of hydrogel thin film attached in a pattern to glass substrate. Furthermore, we showed that a microfluidic device formed by an obtained 3D structure connected to a pump can be used as a cell culture substrate. This highly biocompatible substrate shows properties of deformation and permeability similar to behaviors in an in vivo environment. This method is expected to lead to applications in stem cell-based regenerative medicine and organ-on-a-chip for drug screening, as well as to fundamental technologies for the development of artificial organ models needed for the design of NTT's Bio Digital Twin.
Publication information
Authors: R. Takahashi, H. Miyazako, A. Tanaka, Y. Ueno
Title: Dynamic Creation of 3D Hydrogel Architectures via Selective Swelling Programmed by Interfacial Bonding
Journal: ACS Applied Materials & Interfaces
Date of publication: June 15, 2019 (US time)
Authors: R. Takahashi, H. Miyazako, A. Tanaka, Y. Ueno, M. Yamaguchi
Title: Tough, Permeable and Biocompatible Microfluidic Devices Formed through the Buckling Delamination of Soft Hydrogel Films
Journal: Lab on a Chip
Publication: April 6, 2021 (UK time)
Explanation of experiment
Hydrogel thin film can be chemically attached to a glass substrate by treating the surface of the substrate with adhesion molecules 3-(methacryloyloxy)propyltrimethoxysilane *9. In this study, we used lithography technology to create a pattern of adhesion molecules on the glass surface and synthesized polyacrylamide gel of 30 - 120 μm thickness on the substrate. The hydrogel adheres tightly to the glass substrate in areas where adhesion molecules are present and easily delaminates in areas where adhesion molecules are not present. Swelling is then induced in the hydrogel thin film to deform it to a bent 3D structure (buckling), and the hydrogel delaminates in accordance with the pattern (buckling delamination). In this research, we applied a linear delamination pattern to fabricate a microfluidic structure that is lifted from hydrogel thin film.
Glossary of terms
- *1 ... Hydrogel
- A soft material which retains a large amount of water in networks of polymers. It exhibits properties intermediate between solids and liquids, and characteristics include molecular permeability and volume change from swelling. Because of its high biocompatibility, it is widely used as a biomedical material for applications such as contact lenses, implantable materials in plastic and reconstructive surgery, and cell culture substrates.
- *2 ... Organ-on-a-chip
- Also called a biofunctional chip, an organ-on-a-chip refers to a microfluidic device formed on a chip with the functions of a physiological organ. Using microfabrication technologies, a flow channel scaffold structure is created to culture organ cells and express organ functions. Organs-on-a-chip hold great promise as a technology for implementing clinical experiments without relying on lab animals or human subjects, and is being actively researched with the aim of realizing various artificial organs.
- *3 ... Polyacrylamide gel
- A network structure of many (poly) chemically or physically intertwined low-molecular-weight acrylamides (polyacrylamides), and retains water. A commonly used hydrogel, it is widely used in cosmetic products and implant materials.
- *4 ... Buckling delamination
- A thin film is compressed from both ends to lift the bent central portion, and deforms into an arch structure. This is called the buckling delamination phenomenon.
- *5 ... Myoblasts
- Mononuclear cells (cells with a single nucleus) that differentiate into muscle fibers by assembling and fusing together in large numbers.
- *6 ... Bio Digital Twin
- Supporting the foundation of the NTT Medical and Health Vision, Bio Digital Twin (BDT) technology precisely maps each individual's body in cyberspace.
- *7 ... Growth factor
- Also known as growth factors, growth factors refer to proteins that promote the differentiation and proliferation of specific cells in the body of animals. Because of their distinct physiological effects like regulation of organogenesis, they play an important role in regenerative medicine and organs-on-a-chip.
- *8 ... Acetylcholine
- A neurotransmitter that transmits nerve stimulation inside the body. It binds to acetylcholine receptors in skeletal muscle and cardiac muscle fibers to trigger muscle contraction. It is also sold as a drug to counteract intestinal tract paralysis and acute gastric dilatation after anesthesia.
- *9 ... 3-(Methacryloyloxy) propyltrimethoxysilane
- A type of adhesion molecule called silane coupling agent, it contains functional groups within the molecule that can bind to both organic and inorganic materials. In this research, we used a molecule that has a binding site for glass and other silicon-based materials and a binding site for hydrogel.
Video links
(Video 1) Swelling-driven buckling delamination of hydrogel thin film
(Video 2) Reversible ON-OFF control of structure using thermo-responsive gel
(Video 3) Large deformation and periodic deformation behaviors in thin film device
(Video 4) Cell culture in hydrogel flow channel
(Video 5) Localized chemical stimulation of cells by injection of drug into flow channel